Evolution of immature stages in Muscidae (Diptera)
Immature stages of muscidae
Muscidae exhibits extraordinary diversity in morphology, life history, and behaviour at both immature and adult stage. In muscid immature stages, one can find a wide range of feeding habits, ranging from various types of decomposing organic matter, dung, fungi, through to live invertebrates and vertebrates [1]. The filtering of decomposing material, by means of saprophagy or coprophagy, is present in many species of Muscidae. On the other hand, obligatory carnivores successfully reach maturity only after having access to living prey and this strategy is correlated with the reduction in the number of free-living larval stages [2]. However, a mixed strategy is also present in some lineages and in facultative predators, early larval instars are filter feeding saprophages, while the last instar may reveals predatory behaviour. Some representatives of Atherigona Rondani are phytophages associated with various species of grasses (Poaceae). Subcutaneous parasites of birds nestlings evolved in Passeromyia Rodhain & Villeneuve and Philornis Meinert and endoparasites of millipedes in Eginiini [1]. The larvae of some Azeliini, Muscini and Reinwardtiini can be involved in cases of secondary myiasis in people and animals. On the other hand, some species may be highly beneficial to humans as biological agents of pest control of forensic indicators [3].
In Muscidae, various changes from the cyclorrhaphan ground-plan of the cephaloskeleton in the number, shape, and size of sclerites can be found. For example, in second instars robust mouthhooks with accessory ventral teeth occur in Atherigona that rasp hard, plant material; in second instars of some coprophagous species partial (Fig. 1B) or entire (Fig. 1C) reduction of the mouthhooks is present; in third instars, sharply pointed mouthhooks with a long and slender basal sclerite may be found in obligatory predators (Fig. 1H); an intermediate sclerite is fused with the basal sclerite in plant cutting Atherigona (Fig. 1F); in phytophagous species mouthhooks are reduced and their role is taken by enlarged accessory oral sclerites (Fig. 1F); accessory oral sclerites below the apical part of the mouthhooks are present within lineages revealing facultative and obligatory carnivory (Fig. 1D, H, G, H) [1,4].
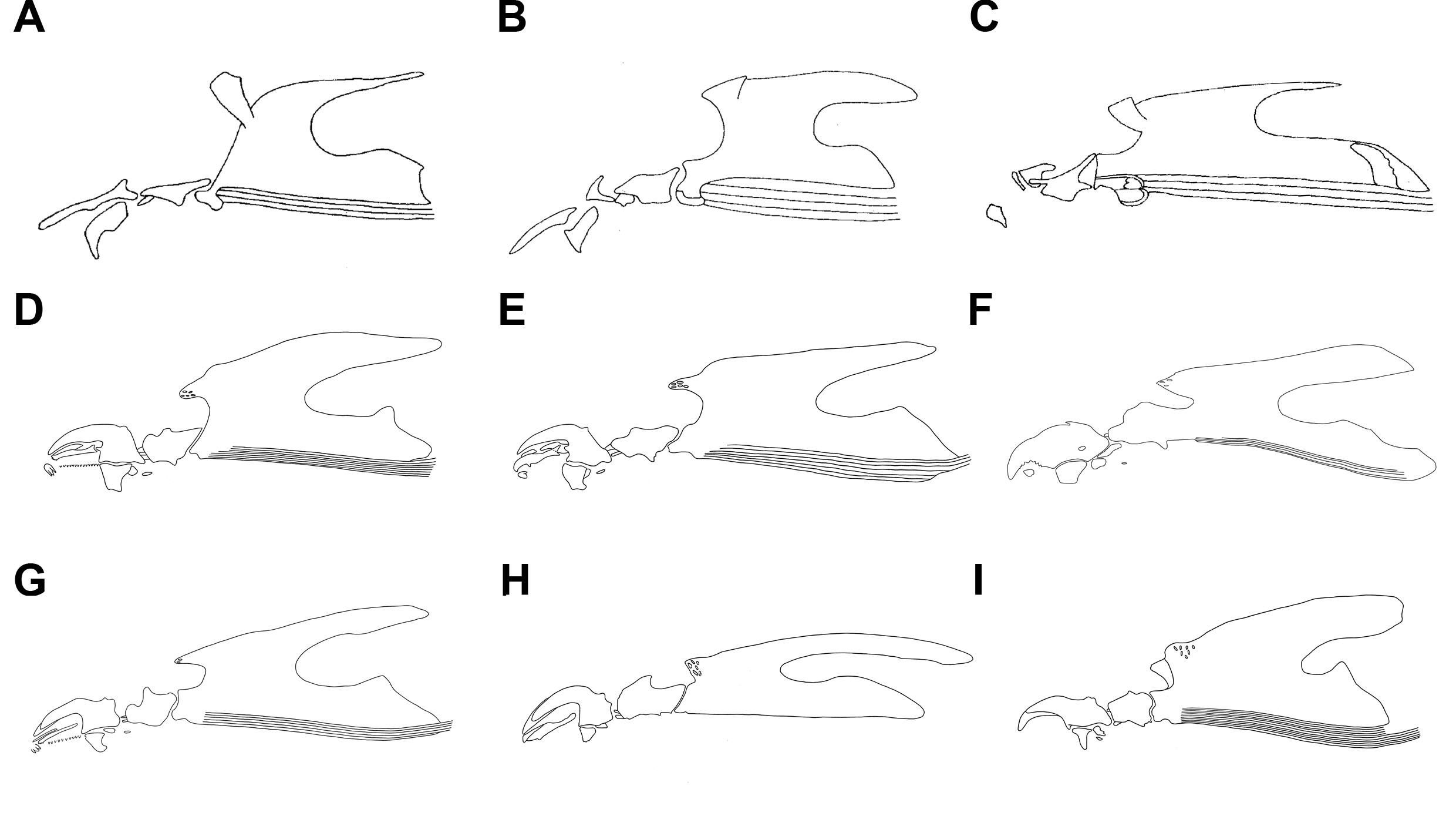
Fig. 1. Examples of details of the cephaloskeleton of second (A–C) and third (D–I) instar larvae of Muscidae: A) Azeliini, facultative predator; B) Muscini, saprophage; C) Stomoxyini, coprophage; D) Reinwardtiini, facultative predator; E) Atherigoninae, facultative predator; F) Atherigoniinae, phytophage; G) Azeliini, facultative predator; H) Azeliini, obligatory predator; I) Muscini, coprophage. A–C from Ferrar (1987).
Facultative predators, in the third instars of the great majority of muscid species known from immature stages are equipped with the highest number of additional sclerites in the cephaloskeleton [1]. According to Hennig [5], such a fully equipped cephaloskeleton is the ancestral state for the family. In this case, the typical cyclorrhaphan simple cephaloskeleton without accessory oral sclerites, which is often found within purely saprophagous Muscidae, should be considered a derived state (Fig. 2A). Consequently, the shift from facultative carnivory to saprophagy should be associated with the secondary reduction and loss of accessory oral sclerites, i.e., a simplification to a form similar to the cyclorrhaphan ground plan. On the other hand, according to Kutty et al. [2], saprophagy, not a mixed strategy, is the ancestral feeding strategy of Muscidae (Fig. 2B). This result is in agreement with the assumption that filtering of decomposing material is an ancestral strategy for immature stages of the Cyclorrhapha [6]. Based on the present state of knowledge of muscid larval morphology [1,3,7,8], under the assumption of Kutty et al. [2] the structure of the cephaloskeleton is most probably not correlated with feeding strategy. According to the results of Kutty et al. [2], in saprophages either the typical cyclorrhaphan ground-plan cephaloskeleton is present, or accessory oral sclerites may occur in the apical part of the mouthhook.
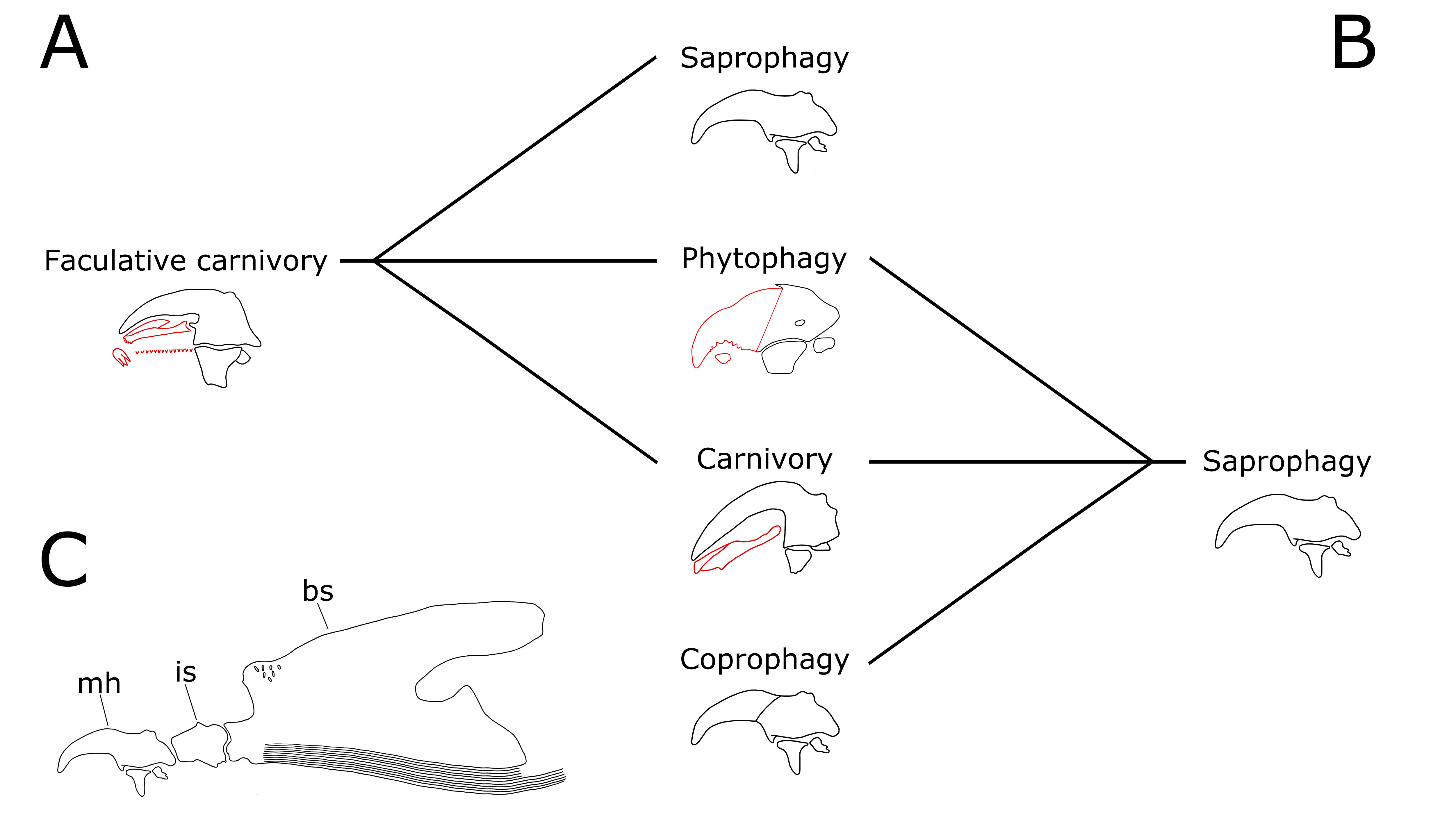
Fig. 2. Evolution of Muscidae feeding strategies and details of larval morphology: A) Shifts to saprophagy, phytophagy, and obligatory carnivory from ancestral facultative carnivory (Hennig 1965); B) Shifts to coprophagy, phytophagy, and carnivory from ancestral saprophagy (Kutty et al. 2014); C) Cyclorrhapha ground-plan of the cephaloskeleton of the third instar larva. In A and B, possible cephaloskeletons are presented for different feeding strategies under a given hypothesis. Abbreviations: bs, basal sclerite, is, intermediate sclerite; mh, mouthhook. Accessory sclerites, below apical part of mouthhooks, are marked in red.
Muscidae phylogeny – state of the art
The interpretation of the Muscidae as a family and their classification into higher-level taxa has been a matter of debate for many years. Previous concepts included in Muscidae taxa that are currently relevant to our understanding of the Oestroidaea. This can be observed, for example, in the number of proposed subfamilies ranging from four, i.e., Fanniinae, Muscinae, Mydaeinae and Phaoniinae (Hennig 1955–1964), to up to 15, i.e., Athomyiinae, Cobboldinae, Coenosiinae, Eginiinae, Fanniinae, Fucelliinae, Gasterophilinae, Glossininae, Gyrostigminae, Lispinae, Muscinae, Phaoniinae, Prosthetosominae, Rutteniinae and Stomoxydinae [10]. The concept of the family as understood today is a relatively recent advantage and is a result of the exclusion of the Fanniinae and elevating the group to the family level and a transfer of the genus Coenosopsia Malloch to Anthomyiidae [11]. Significant progress in higher-level classification has been made with the advent of morphological data analysis in realms of phylogenetic concepts [e.g., 5,12–15]. Incorporation of molecular data was another breakthrough in the understanding of relationships within the family [2,16–21]. The current concept of the Muscidae was widely supported with molecular data. Many results have been consistent with morphology-based hypotheses, particularly the classificatory system proposed by Fan [22]. While recent results from mS-seq, AHE and RAD-seq analyses are at odds with adult morphology-based classifications [2,16–21], are partially supported by immature stage morphology and natural history. Some morphological characters from adults, considered for a long time of primary importance for classification of Muscidae, are currently recognized as results of convergent evolution, as in the case of the shape of wing veins, presence of setae on the hind coxa or presence of spines on the inner surface of the cercal plate in males.
In our work, congruence among various molecular approaches will be used to build confidence for particular phylogenetic hypotheses. Morphological data from adult and will be used as corroboration of certain nodes in our phylogenetic trees in order to build a new robust classification system for the Muscidae.
references
1. Skidmore P. 1985. Ser. Entomol. 29, 1–550.
2. Kutty SN, Pont AC, Meier R, Pape T. 2014. Mol. Phylogenet. Evol. 78, 349–364.
3. Grzywacz A, Hall MJR, Pape T, Szpila K. 2017. Int. J. Legal Med. 131, 855–866.
4. Ferrar P. 1987. Entomonograph 8, 1–907.
5. Hennig W. 1965. Stuttgarter Beiträge zur Naturkd. 141, 1–100.
6. Marshall SA. 2012 Flies : the natural history and diversity of Diptera. Buffalo, New York: Firefly Books.
7. Grzywacz A, Pape T. 2014. Acta Trop. 137, 174–184.
8. Grzywacz A, Lindström A, Hall MJR. 2014. Forensic Sci. Int. , e34–e43.
9. Hennig W. 1955 63 b. Muscidae. In Die Fliegen der Paläarktischen Region (ed E Lindner), pp. 1–1110. Stuttgart: Schweizerbart’sche Verlagsbuchhandlung.
10. Séguy E. 1937 Diptera Fam. Muscidae. In Genera Insectorum, vol 205 (ed P Wytsman), pp. 1–604. Bruxelles: Desmet-Verteneuil.
11. Michelsen V. 1991. Syst. Entomol. 16, 85–104.
12. Couri MS, Pont AC. 2000. Syst. Entomol. 25, 373–392.
13. Savage J, Wheeler TA. 2004. Stud. Dipterologica 11, 259–300.
14. Nihei SS, Carvalho CJB. 2007. Zool. J. Linn. Soc. 149, 493–532.
15. Carvalho CJB de. 1989. Rev. Bras. Zool. 6, 627–648.
16. Schuehli GSE, Carvalho CJB de, Wiegmann BM. 2007. Invertebr. Syst. 21, 263–278.
17. Haseyama KLF, Wiegmann BM, Almeida EAB, de Carvalho CJB. 2015. Mol. Phylogenet. Evol. 89, 1–12.
18. Grzywacz A, Wallman JF, Piwczyński M. 2017. Syst. Entomol. 42, 714–723.
19. Kutty SN et al. 2019. Cladistics 35, 605–622.
20. Junqueira ACM, Azeredo-Espin AML, Paulo DF, Marinho MAT, Tomsho LP, Drautz-Moses DI, Purbojati RW, Ratan A, Schuster SC. 2016. Sci. Rep. 6, 21762.
21. Grzywacz A, Trzeciak P, Wiegmann BM, Cassel BK, Pape T, Walczak K, Bystrowski C, Nelson L, Piwczyński M. 2021. Syst. Entomol. 46, 508–525.
22. Fan Z. 2008 Diptera Muscidae (1). Fauna Sin. Insecta 49, 1–1180.